
Mohammad Abuannadi, MBBS
Amyloid/Sarcoid Clinical Program Update
Amyloidosis is a condition caused by the deposition of misfolded proteins in tissues including the heart, nerves, and kidneys. Cardiac amyloidosis has been considered a rare disease but recent data have suggested that the disease is more common than previously thought. In addition to increased recognition of the disease, there have been significant advances in the evaluation and treatment of amyloidosis. Patisiran (Onpattro) is an RNA interference therapeutic agent that inhibits the hepatic synthesis of transthytretin, and in patients with hereditary transthyretin amyloidosis with polyneuropathy, improves symptoms. More recently, tafamadis (Vyndaqel and Vyndamax) a transthyretin tetramer stabilizer, has been shown to lower all-cause mortality and slow the progression of symptoms of ATTR cardiac amyloidosis. In addition, other medications are currently undergoing evaluation for treatment in this growing field.
Given the systemic nature of amyloidosis and the complexity of the evaluation, we started a multidisciplinary collaboration with colleagues from heart failure, cardiac imaging, hematology, genetics, neurology, and hand surgery in order to improve the quality of care provided to patients with suspected or confirmed cardiac amyloidosis. One of the goals of our collaboration is to become a regional center of excellence for the care of patients with amyloidosis and to utilize research to help advance the field.
Sarcoidosis is another systemic disease that can involve different organs including the heart and lungs. In order to optimize the care of patients with this disease, a collaboration between pulmonology (Dr. Catherine Bonham) and heart failure cardiology (Dr. Mohammad Abuannadi) is ongoing. One of the goals of the collaboration is to launch a combined pulmonary- cardiac sarcoidosis clinic in the very near future, where specialists from both disciplines see patients together. In addition to the collaboration with pulmonology, the University of Virginia program offers the advantage of a nationally recognized cardiac imaging program that is essential for accurate diagnosis and guiding therapy of cardiac sarcoidosis. Another area of strength is the collaboration with electrophysiology team that provides excellent support to manage the arrhythmias that can complicate cardiac sarcoidosis. In addition to providing excellent clinical care to the patients, advancing the field of sarcoidosis through research is one of the important long- term goals of our program.
Please feel free to call or email Dr. Mohammad Abuannadi (913-484-2154; MA3WN@hscmail.mcc.virginia.edu) with inquiries or referrals of patients with suspected or confirmed cardiac amyloidosis or cardiac sarcoidosis.

Linda Bailes Bryceland, RN, CCRC
Bailes Bryceland Research
By Linda Bailes Bryceland, RN, CCRC
Our CIC research team is coordinating 2 Covid-19 PI principal investigator-initiated UVA trails.
UVA Biorepository study is Uva PI initiated study collected prospective lab samples on Covid-19 positive patients at 4-time points during their hospital stay. This involves getting consent and ordered the lab samples in Epic and working with the nurses on the special pathogens unit to coordinate the collection of the samples and the BTRF staff to collect and test and store all the lab samples.
The study is also collecting all “leftover or discarded samples” for all labs on COVID-19 patients. These samples are stored and tested in BTRF lab here in MR6 at UVA.
Our second study is the UVA Phase II COVID-19 Convalescent Plasma treatment study. This is a treatment study using donated plasma from recovered Covid-19 patients as a treatment in our currently admitted Covid-19 patients in hopes of preventing the progression of their illness and hopefully prevent their admission to the ICU and requiring ventilator use.
Our team is coordinating all activities with this study. We consent patients, order their convalescent plasma, and work with the Special Pathogen Unit nurses on plasma infusions and collection of follow up lab samples, and data on the patient’s condition.
Our entire staff has been working directly with the UVA COVID-19 special research committee, iTHRIVE team, IRB, BTRF, and the staff on the special pathogens unit to coordinate and run these studies.

Kenneth Bilchick, MD
Bilchick Research
Kenneth Bilchick, MD, Director of Electrophysiology Research at the University of Virginia Health System, is very interested in using advanced cardiac imaging to improve outcomes for patients with arrhythmias and heart failure. In particular, his group has an ongoing randomized clinical trial evaluating the intervention of cardiac magnetic resonance (CMR) to guide the selection of left ventricular pacing sites for patients undergoing cardiac resynchronization therapy (CRT) implants. Dr Bilchick has also developed and validated innovative methods for imaging patients with CRT devices in the MRI scanner to obtain high-quality data regarding improvement in biventricular function after resynchronization.
In collaboration with Frederick Epstein, PhD and Jeffrey Holmes, MD, PhD in the Department of Biomedical Engineering and industry partners, he also has ongoing studies evaluating mechanisms of CRT response using bioengineering models of heart failure based on electromechanical parameters derived from CMR and body surface electrical potentials.
This work on heart failure mechanisms has also been complemented by exciting work with Coleen McNamara, MD’s lab on inflammatory pathways in heart failure, in which peripheral blood mononuclear cell gene and protein expression are analyzed using RT-qPCR and CyTOF, respectively.
In addition, Dr Bilchick continues to have productive outcomes projects with the National Cardiovascular Data Registry to evaluate outcomes after ICDs at the national level and also collaborative work with Sula Mazimba, MD on clinical outcomes of UVA patients with cardiovascular disease, including a very interesting recent analysis of the impact of longitudinal trajectories of structural and neurohormonal trajectories on survival in heart failure.

Colleen McNamara, MD
Pivoting from CV Research to COVID-19
One fundamental and critical unanswered question in the COVID-19 pandemic is why some individuals with COVID-19 have mild-moderate or no symptoms and others develop cytokine release syndrome (CRS) and have severe courses and death. CRS or cytokine storm (CS) has been clearly linked to COVID-19 disease severity. Levels of circulating cytokines such as IL-6, IL-1ß IP-10, MCP-3, IL-2 are significantly higher in patients requiring ICU care, mechanical ventilation, and those who die from COVID-19. As such, therapies that block the immune cell signaling and activation induced by IL-6 and IL-1ß are currently in clinical trials in COVID-19 subjects at UVA and elsewhere to reduce morbidity and mortality. Yet, strategies for early identification of those at risk of CRS and those who might benefit from these therapies are lacking. Waiting until the patient develops high circulating levels of IL-6 and IL-1ß before initiating therapy may be too late for maximally effective intervention and yet initiating therapy in all COVID-19 subjects may be cost-prohibitive and lead to untoward effect.
Hema Kothari PhD (Assistant Professor of Research in the Cardiovascular Division) working with Chantel McSkimmings, MD/PhD student Oom Pattarabanjird and BME PhD student Corey Williams in the laboratory of Dr Coleen McNamara has been studying the immune cell signaling induced by IL-6 and IL-1ß in humans. Drugs that target these pathways are approved for therapy for autoimmune diseases and are emerging as potential therapies for cardiovascular diseases (CVD). Utilizing mass cytometry (a cutting edge technology available at a limited number of institutions including UVA) to analyze human circulating peripheral blood mononuclear cells (PBMCs), she has identified the cell types most vulnerable to IL-6 and IL-1ß activation and their signatures (Figure 1).
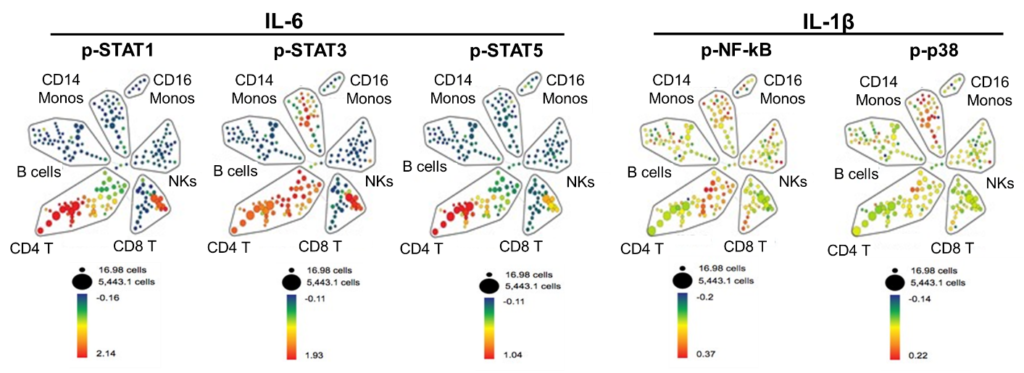
Figure 1: Spanning-tree Progression Analysis of Density-normalized Events (SPADE) demonstrates that CD4 and CD8 T cell subtypes are the circulating human immune cell subtypes most vulnerable to IL-6-induced activation of STATs. In contrast, IL-1β induces activation of NF-kB most prominently in a subset of CD4 T cells and of p38 in the CD14 monocytes. Human PBMCs were stimulated with vehicle control or IL-6 and IL-1β for 15 minutes. Representative SPADE analysis using cell surface markers to identify immune subtypes as labeled and color (blue to red) corresponds to the fold change in the median expression of p-STATs, p-NF-kB and p-p38.
Notably, she discovered consistency in IL-6 and IL-1ß-induced-activation with repeat testing in the same individual, yet a heterogeneity inactivation from one person to the next. In some individuals, stimulation with IL-6 and IL-1ß robustly induces phosphorylation of signaling intermediates (such as NF-KB, p38 and STAT1, 3 and 5) that are critical for induction of further cytokine production (cytokine storm) and in other individuals there are much lower levels of activation. As NF-KB and STATs induce downstream cytokine production, it is likely that those with robust IL-6 and IL-1ß-induced activation of these intermediates will have greater inflammation and a more severe clinical course. It is also likely that these individuals will have a better response to therapies that block these cytokine signaling events. We are currently testing this hypothesis in a clinical trial of Anakinra (an IL-1ß receptor blocker) for heart failure. Thus, our customized panels for quantitating IL-6 and IL-1ß activation in human immune cells and all the necessary equipment, personnel, methods, and data analytics were on hand and “shovel ready” to pivot to COVID-19 to address the critical unanswered questions of how to achieve early identification of those at risk of CRS and identify those that might respond to agents that block CRS. In addition to studying COVID-19 patients with mild-moderate versus severe clinical courses at UVA to identify a precision biomarker of disease severity, we are also studying COVID-19 patients enrolled in the Remdesivir-Baricitinib trial to identify a signature that might predict Baricitinib therapy responders to potentially provide for future personalized medicine approaches.

Zhen Yan, PhD
Yan Lab
Regular exercise has profound health benefits and is the most powerful intervention in disease prevention and treatment. Our lab focuses currently on elucidating the molecular mechanism by which aerobic exercise promotes the removal of damaged/dysfunctional mitochondria through mitophagy, a self-eating process of mitochondria, in skeletal muscle and heart.
Our research led to a new discovery of mitochondria-associated AMP-activated protein kinase (AMPK) and its regulatory role in the control of mitophagy. We are taking advantage of CRISPR-Cas9-mediated gene editing to further dissect the molecular mechanisms of this important regulator that is critical for exercise-mediated protection against diabetes and heart failure.
We are also investigating the role and mechanism of early exercise intervention in the prevention of the symptomatic onset of Friedreich’s ataxia, an inherited mitochondrial disease. In our ongoing research, we have for the first time shown that long-term aerobic exercise training in a mouse genetic model of Friedreich’s ataxia could completely prevent the symptomatic onset of the functional and biochemical abnormalities caused by reduced frataxin gene expression.

J. Randall Moorman, MD
The Center for Advanced Medical Analytics
The Center for Advanced Medical Analytics, run by Randall Moorman and Doug Lake with the recent addition of Oliver Monfredi, tests hypotheses about signatures of illness prior to clinical deterioration and develops decision support tools that convert readily available clinical data into risk estimates for bedside use. Much of the work is to study disordered breathing in premature infants, and they serve as the Data Coordination Center for a U01 grant. Their work is undertaken with medical students to senior faculty elsewhere in the Department (ID, Critical Care, General Medicine), other Departments (Surgery, ED, Pediatrics, Neurology), Schools (Nursing, Engineering, Data Sciences) and institutions. Recent work includes the application of thousands of numerical algorithms to TB of continuous monitoring and clinical data to generate a library of findable, accessible, interoperable, and reusable data and results that is available to UVa researchers.
Future work includes a randomized clinical trial of predictive analytics monitoring on the 4th floor (Jamie Bourque, coPI).
Through a commercial partner*, CoMET predictive analytics monitors will be installed in several locations throughout UVa Hospital.
(*AMP3D – Moorman has COI, as he is CMO and owns equity.)
Filed Under: Clinical Research, News and Notes
